2 min read
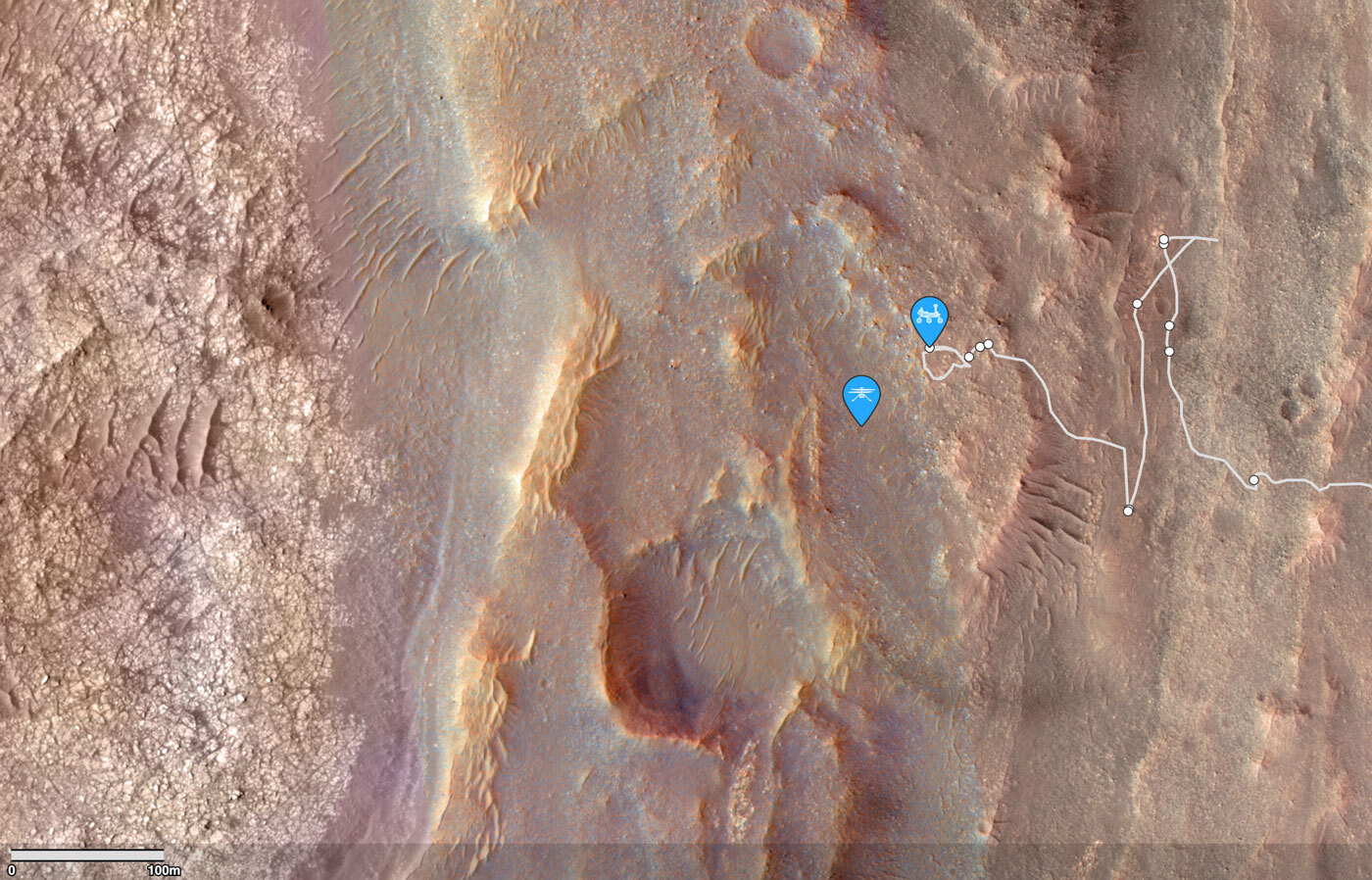
After more than two and a half years of driving and exploring, Perseverance is closing in on an eagerly anticipated destination: the margin carbonate unit.
The Mars 2020 scientists have been buzzing with excitement this past week as Perseverance makes its final approach towards a special rock unit that played a pivotal role in selecting Jezero as the landing site for exploration. Located in a narrow band along the inner edge of Jezero’s western crater rim, this layer showcases pronounced signatures of a mineral known as carbonate. On Earth, carbonates typically form in the shallow shoals of freshwater or alkaline lakes. It's hypothesized that this might be the case for the margin carbonate unit on Mars too—over 3 billion years ago, the waters of a lake in Jezero crater might have lapped against its shores, depositing this carbonate layer. An alternative hypothesis is that the carbonates formed through mineral carbonation, where silicate minerals (like olivine) react with CO₂ and are converted to carbonates.
Carbonates are intriguing for several reasons. Firstly, carbonates can offer insights into Mars' bygone atmosphere. These minerals form through a series of chemical reactions that begin when carbon dioxide (CO₂) from the atmosphere reacts with liquid water. Thus, by studying the presence, abundance, and isotopic composition of these carbonates, our team may be able to infer Mars’ past atmospheric CO₂ levels and glean insights into its climatic history.
Second, carbonate minerals are an excellent medium for preserving traces of ancient life if it existed. When carbonates precipitate early in the rock-forming process, they can capture a snapshot of the environment in which they formed, including any signs of microbial life. On Earth, carbonate minerals have been observed to form directly around microbial cells, encapsulating them and rapidly turning them to fossils. This is particularly valuable because once an organism is encased in carbonate it can be preserved for a very long time. Another example of carbonate fossilization on Earth is stromatolites—layered structures created by microbial colonies growing in mineral-saturated waters. Stromatolites represent some of the earliest records of life on Earth.
Although we don’t yet know exactly how the margin rocks, or the carbonate within them formed, the team is eager to drill into these rocks and unlock their secrets.
Written by Erin Gibbons, Student Collaborator at McGill University